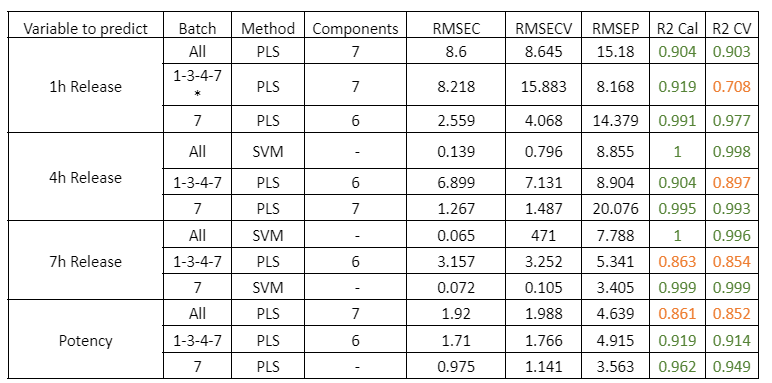
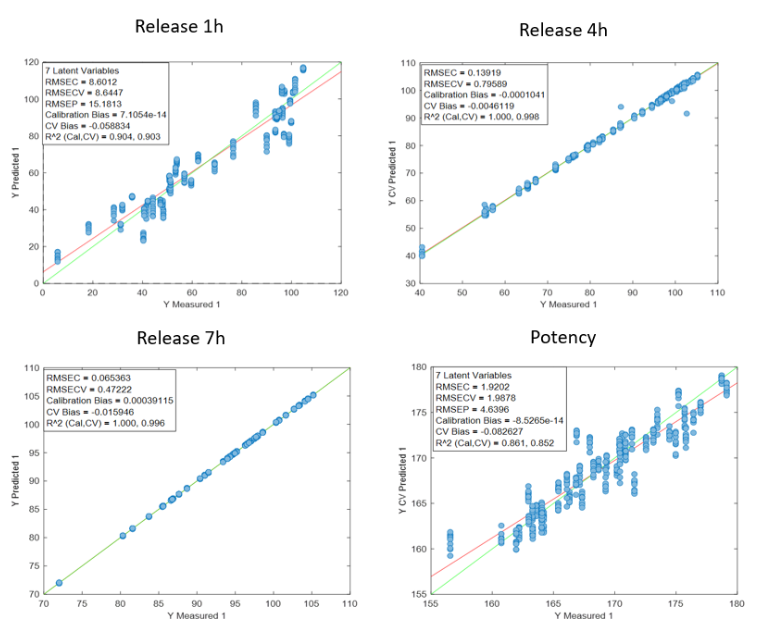
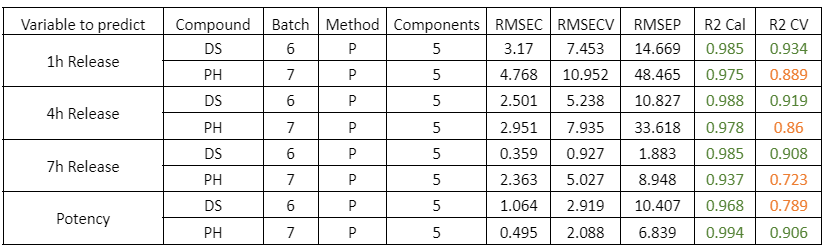
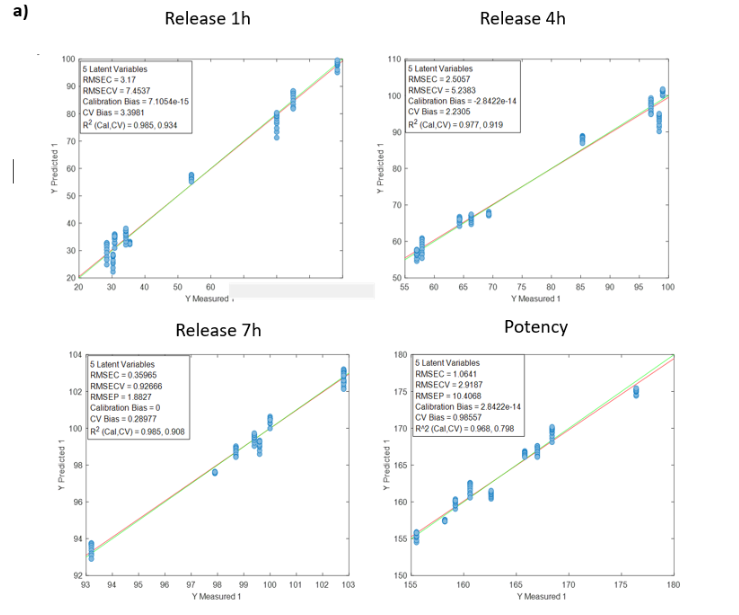
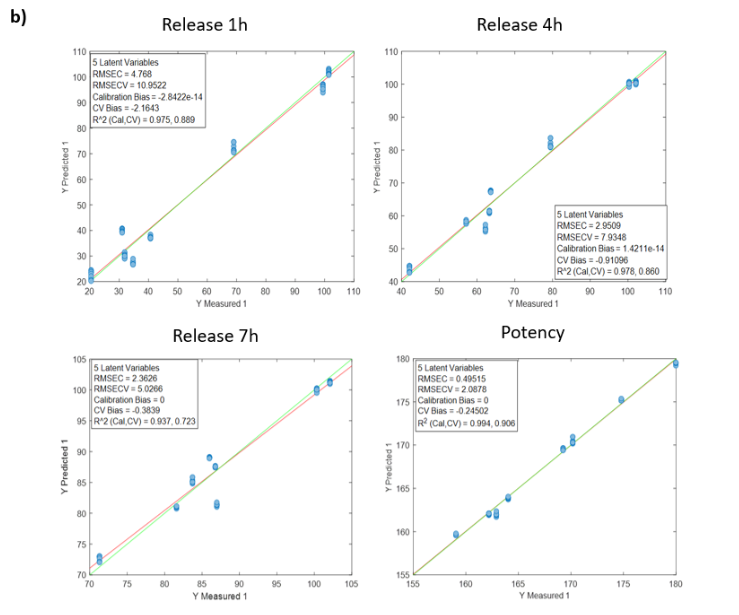
Visum Raman In-Line™ ile sakızların gerçek zamanlı pişme derecesi takibi
Visum Raman In-Line™ ile sakızların gerçek zamanlı pişme derecesi takibi
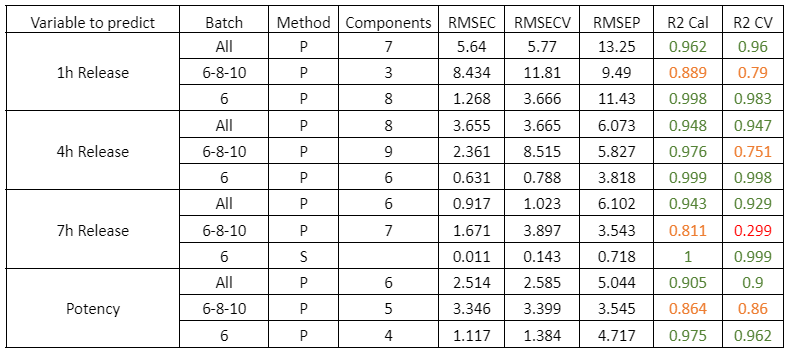
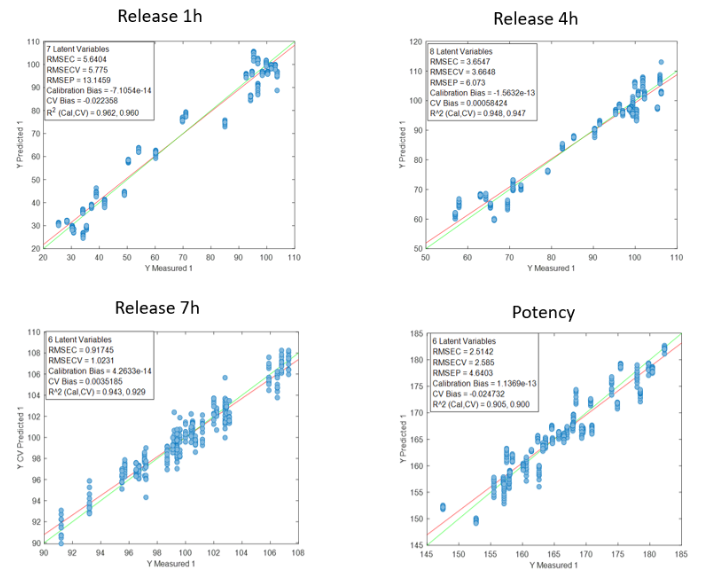
 Blending process monitoring with in-line NIR spectroscopy
Blending process monitoring with in-line NIR spectroscopy

European Commission Highlights IRIS Technology Solutions for its Innovative Role in the AIDPATH Decentralized Advanced Therapy Production Project

IRIS Technology Solutions is proud to announce that we have been recognized as a “Key Innovator” by the European Commission’s Innovation Radar for our contributions to the AIDPATH project. This recognition highlights our innovative development of a monitoring strategy for optimal measurement of selected parameters in bioreactors, leveraging cutting-edge technologies such as Artificial Intelligence (AI), soft-sensors, and machine learning algorithms.
AIDPATH (Artificial Intelligence-driven, Decentralized Production for Advanced Therapies in the Hospital) is a high-impact EU-funded consortium dedicated to advancing the next generation of personalized medicine using gene-engineered immune cells at EU hospitals through AI technology. The project focuses on T cells modified to express a synthetic chimeric antigen receptor (CAR-T), a revolutionary treatment in hematology and oncology, with potential applications for infections and autoimmune diseases. AIDPATH aims to address the complexities of traditional CAR-T therapy, which is hindered by centralized manufacturing and inflexible clinical use schemes. By integrating patient-specific data and biomarkers, AIDPATH uses AI to enable flexible manufacturing and optimize CAR-T cell products, improving anti-tumor potency and reducing costs and resource utilization in hospitals.
Advanced Monitoring Strategy for Bioreactors
In this project, IRIS Technology Solutions played a pivotal role by developing a monitoring strategy that allows for the optimal measurement of selected parameters in bioreactors. Bioreactors are essential in bioprocessing as they provide the environment needed for biological reactions to occur, such as for the production of pharmaceuticals, biofuels and other bio-based products. In the case of AIDPATH, the bioreactor performs a so-called “perfusion” process which, starting from a small number of CAR T-cells taken from a patient, grows a much larger amount, which are then reintroduced into the patient for immunotherapy cancer treatment. However, maintaining optimal growth conditions within the bioreactor is a complex task that requires precise monitoring of variables such as pH, temperature, oxygen levels, and nutrient concentrations.
We tackled this challenge by designing a system capable of monitoring these parameters in real time, adapting dynamically to changes and ensuring that the bioreactor’s conditions remain optimal for the biological processes involved. One of the key achievements was our development of a control system that provides alerts based on the variations in these parameters. The system was programmed to follow specific control rules and thresholds to ensure expected behaviors during in-line monitoring, making it more efficient and reliable than traditional systems.
Innovation through AI-Powered Soft-Sensors
A standout innovation that contributed to this recognition was our integration of AI-powered “smart-sensors” within the monitoring system. Unlike the traditional approach that provides information based on a small number of hard sensors with a PID controller, our smart-sensors apply AI technologies (Fuzzy and Consensus Based algorithms) and advanced statistical techniques (Bollinger, sliding window), to aggregate the data from a larger and more varied set of hard sensors and convert it into actionable insights.
These smart-sensors have been designed to support decision-making by providing real-time insights into the bioreactor’s performance. For instance, instead of simply reporting temperature fluctuations, the soft-sensors analyze the behavior over time and relate it to the trends from other hard sensors. This approach allows for a more holistic feedback to the human expert operator of any significant changes within the bioreactor, ultimately leading to more efficient bioprocesses and higher yields.
Additionally, the soft sensors are integrated in the SCADA type interface (called COPE in AIDPATH) which displays the soft sensor outputs and alerts in real time on a dashboard which is easily interpretable by the human expert operator. Furthermore, the smart sensors are completely parameterizable (soft coded) by the end user, an advantage when calibrating the system for new use cases. Data analytics and machine learning can be used to further fine tune the control parameters and data processing rules.
Being recognized by the European Commission’s Innovation Radar underscores IRIS Technology Solutions’ leadership in advanced process monitoring and AI applications. This recognition not only acknowledges our efforts but also opens opportunities for future collaborations and partnerships with other innovators, businesses, and academic institutions. It highlights our commitment to developing cutting-edge technologies with real-world applications and impact.
Moreover, the Innovation Radar platform provides visibility to potential customers, investors, and partners seeking innovative solutions in the biotechnology and pharmaceutical sectors. As part of this platform, IRIS is poised to attract new interest and expand its network within the scientific and industrial communities.
Being named a “Key Innovator” by the European Commission’s Innovation Radar is a significant achievement that reflects our dedication to technological advancement and innovation, as well as the hard work and commitment of our team. We look forward to continuing to drive innovation in advanced process monitoring, artificial intelligence, and beyond.