
 Raman Process Analyzer: Innovation in Industrial Process Control
Raman Process Analyzer: Innovation in Industrial Process Control
 NIRS analysis of Brix degrees in whole apples in real time
NIRS analysis of Brix degrees in whole apples in real time

Quantitative analysis of pharmaceutical forms using NIR spectroscopy

Visum Palm™ Handheld NIR Analyser - Quantitative analysis of pharmaceutical forms
Near infrared spectroscopy (NIRS) is an analytical technique that has gained prominence in the pharmaceutical industry due to its ability to perform rapid, non-destructive analysis without the need for extensive sample preparation. The Visum Palm™ analyser covers the wavelength range between 900 nm and 1700 nm and is characterised by energy absorption due to overtones and combinations of the vibrations of molecular bonds.
This property is particularly useful in the quantification of active ingredients and excipients in solid, liquid or powdered pharmaceutical formulations and content uniformity is undoubtedly one of the most relevant applications of NIRS technology in this industry, replacing or complementing traditional analytical methods in specific applications with real-time process monitoring technologies, a new paradigm called Process Analytical Technologies or PAT.
About the Visum Palm™ Analyser Range - Quantitative analysis of pharmaceutical forms
The spectral range of 900 nm to 1700 nm offers the best trade-off between versatility, maturity and the technology’s ability to work with solid, powder and especially liquid forms. Unlike other analogue devices operating in the 1600 nm to 2500 nm range, the Visum Palm™ spectral range features lower water absorption levels, better light penetration and, consequently, a clearer, more informative signal with lower noise.
Visum Palm™ handheld or benchtop (lab) analyser
Traditional Analytical Methods and NIR Spectroscopy
NIR technology is ideally suited to complement or replace traditional analytical methods such as high performance liquid chromatography (HPLC), UV/Vis spectrophotometry, gas chromatography or chemical titration methods.
A very widespread case in industry is the replacement by NIRS of the Karl-Fischer method for the determination of loss-on-drying (LOD) in pharmaceutical forms. Despite the obvious advantages offered by the NIRS technique (little or no sample preparation, no reagents required, immediate results and possibility to correct process parameters), it is worth mentioning that it does not have the same sensitivity to quantify active ingredients (APIs) or excipients in concentrations typically below the range of 0.1% to 1% by weight, this being the limit of quantification (LOQ) in most cases and quantification applications.
Visum Palm™: Regulatory Compliance
The Visum Palm™ analyser and its software was designed to comply with all recommendations of the American Pharmacopoeia (USP, 1119), the European Pharmacopoeia (Ph. Eu., section 2.2.40), FDA regulation 21 CFR Part 11 and the European Medicines Agency (EMA) Guidelines: ‘Guideline on the use of near infrared spectroscopy by the pharmaceutical industry and the data requirements for new submissions and variations’ (2014), Addendum ‘Defining the Scope of an NIRS Procedure’ (2023), as well as the recent ‘ICH Q14 Guideline on analytical procedure development’ of December 2023.
- CFR compliance and traceability of all device usage information. Audit Trail.
- Verification of photometric wavelength, noise and linearity. Guided wizard from the analyser. Automated device status reporting.
- Self-diagnostics.
- Ability to develop, update and edit identification, classification and quantification methods without expert knowledge.
- Automated reports with technical information of the developed or edited NIRS methods as information to detect improvement needs, maintenance plan or for the validation dossier.
- Possibility to validate NIRS methods with external samples offline.
- Customisation of usage reports for identification or quantification analysis.
- User and permissions management. “Analyst”, “Supervisor”, User Administrator”.
Visum Palm™ handheld analyzer
Benefits of NIR Spectroscopy in Quantification
- Speed of analysis
NIR spectroscopy offers significantly shorter analysis times compared to traditional methods. NIR analysis can be performed in a matter of seconds, enabling real-time quality control during production.
- Non-destructive analysis
Unlike most traditional methods, which require sample destruction, NIR spectroscopy can be performed directly on tablets, powders or solutions without disturbing them, allowing higher sample throughput for future analysis. Even directly through translucent bags.
- Little or no sample preparation
The NIR technique allows direct analysis of the formulation, eliminating laborious sample preparation steps such as dissolution, filtration or dilution, thus reducing the risk of errors and excessive handling.
- Cost reduction
By eliminating the need for solvents, reagents and other consumables, the NIR technique can significantly reduce the costs associated with routine analysis. In addition, its ease of automation decreases the manual labour time required, significantly reducing cycle times and lead time.
- Multiparametric
NIR spectroscopy allows simultaneous quantification of multiple parameters in a sample or formulation. With a properly calibrated method, the concentration of different APIs, excipients and even other properties can be determined in a single analysis.
- Integration in manufacturing processes
The NIR technique is easily integrated into process monitoring systems (PAT), allowing in-line or real-time control of formulation properties during manufacturing, thus improving process efficiency.
Development of a quantitative method with Visum Palm™.
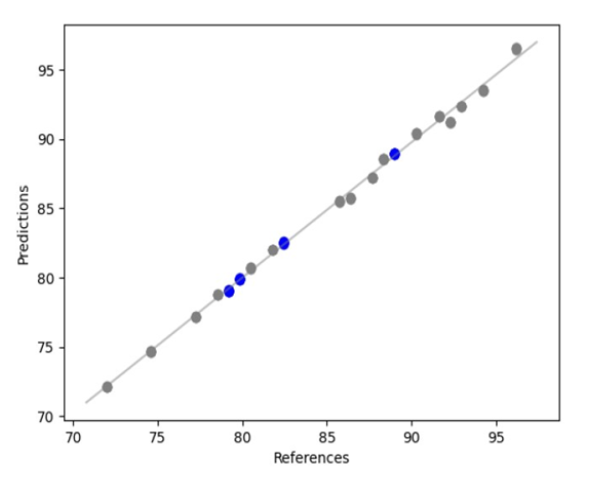
Below we show a case of use of the Visum Palm™ analyser for quantification (content uniformity test) in a pharmaceutical formulation where the active ingredient ‘X’ has a target concentration of 80% w/w.
For this purpose, a set of 20 calibration samples with sufficient variability in terms of API concentrations between 72% and 96% respectively were available for the development of the predictive model.
The Model Builder Visum Master™ as shown below, generates an automatic 80/20 split of the calibration and internal validation sample set.
API ‘X’ regression curve. Calibration or modelling samples (grey) and internal validation samples (blue).
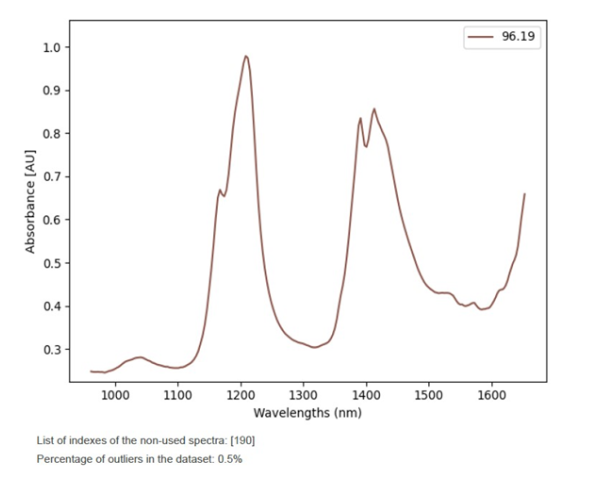
Another important automatic feature is the execution of an automatic quality routine for the detection of outliers, which are spectra that are not found within the model space.
The Visum Master™ software plots, identifies, eliminates and quantifies the spectral outliers found in the modelling set. In this case, less than 0.5%, which is a good indicator of the quality of the spectral information entered.
In any case, it is not recommended to use a model with more than 10% of outliers detected and consequently the process of sample preparation and spectrum acquisition should be reviewed.
Outliers detected and removed from the modelling sample set
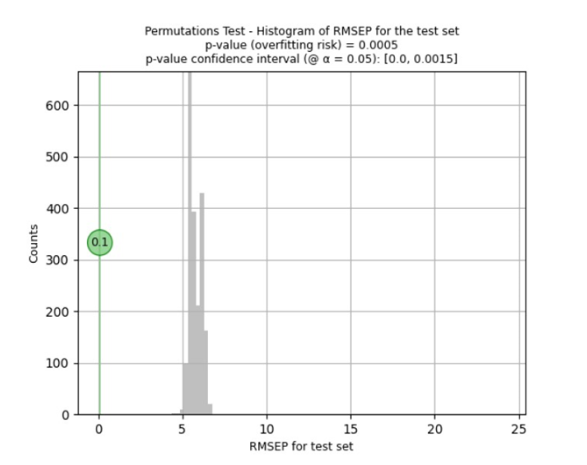
To determine the risk of overfitting, i.e. that the applied pre-treatments and pre-processing result in an apparently good model that does not necessarily work with new and future samples, the Visum Master™ software runs the Fisher-Pitman permutation test to ensure its quality for working with future samples.
Conclusions: Quantitative analysis of pharmaceutical forms using NIR spectroscopy
The resulting predictive model for the quantitative determination of API ‘X’ yielded a correlation coefficient (R²) of 0.99 and a Root Mean Squared Error of Prediction (RMSEP) of ± 0.1










